A new horizon in neurodegenerative
disease treatments
Our mission is clear:
Advancing groundbreaking science to reach a new horizon in neurodegenerative disease treatments.
We’re focused on optimising quality of life for people living with neurodegenerative diseases and moving forward at pace to make innovative treatments available sooner.
Our scientific expertise is driving this momentum. After a decade of research and development, we are pioneering novel applications for NUZ-001 (S-Monepantel).
Long-term safety, tolerability, and strong signals of efficacy point to the real possibility that a life-changing new treatment for Amyotrophic Lateral Sclerosis (ALS) is on the horizon.
Our work also holds promise for other neurodegenerative diseases, including Alzheimer’s, Parkinson’s and Huntington’s Disease, opening new possibilities for millions of patients worldwide.

Amyotrophic Lateral Sclerosis (ALS)
ALS is a devastating neurodegenerative condition that primarily affects nerve cells that control voluntary muscles. It is characterized by severe debilitating symptoms, including muscle weakness, wasting, and paralysis. The limited treatment options available can only manage symptoms, but they cannot reverse the progression of the disease.
We’re working to bring transformative treatments to people living with ALS as quickly as possible

What’s on the horizon right now
There is no time to keep the people living with ALS waiting.
In Australia, we’re exploring compassionate use under the Special Access Scheme, and early access programs in the US and Europe.
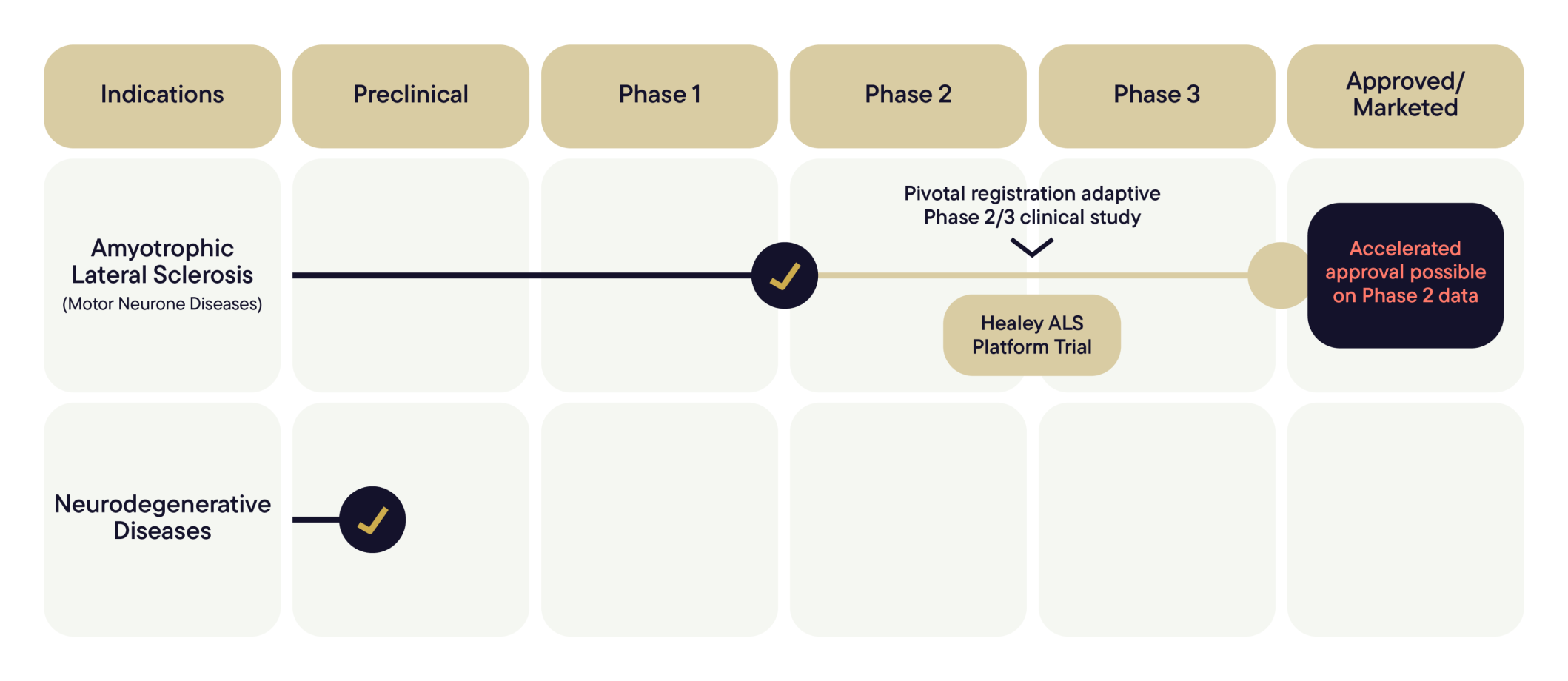
In partnership with the world-renowned Massachusetts General Hospital, we’re entering the Healey ALS Platform Trial with the prospect of accelerated approval from the US FDA and the potential to deliver on our goal of launching our treatment worldwide in the near future.
Product Pipeline
At Neurizon, we’re dedicated to revolutionizing the treatment of neurodegenerative diseases. Our innovative approach is centered on NUZ-001 (S-Monepantel), which has shown promising results in preclinical and clinical studies.

Here’s a glimpse into
our journey:
Preclinical Success
NUZ-001 (S-Monepantel) has shown significant potential in preclinical models, demonstrating its capacity to target and address the underlying mechanisms of neurodegenerative disease.
Phase 1 and Open Label Extension Study Success
NUZ-001 has completed a Phase 1 clinical study and subsequent 12-month open-label extension (OLE) in a small cohort of people living with ALS in Australia. Across the study period, treatment appeared safe and generally well tolerated, with no treatment-related serious adverse events reported. Top-line findings from the Phase 1 and OLE studies demonstrated encouraging trends across exploratory clinical measures, including survival, functional decline, respiratory outcomes, and biomarkers.
Healey ALS Platform Trial
NUZ-001 has been accepted as Regimen I within the HEALEY ALS Platform Trial, following regulatory clearance. Trial start-up activities are progressing, with site activation underway across participating centres and enrolment preparations advancing. Patient enrolment and first dosing are anticipated to commence in Q1 2026, subject to site readiness and regulatory processes.
Together, we’re working to make hope a reality for people living with neurodegenerative diseases
Our Team
Board of Directors
Management
Contact us
Email us: enquiries@neurizon.com
Call us: +61 (3) 9692 7222
Join us on our journey














